| 1. | Introduction | ||||
| 2. | Goals | ||||
| 3. | Program
Design & Usage
|
||||
| 4. | Implementation | ||||
| 5. | Evaluation & Feedback |
![]()
Application of Interactive Web Tools in Teaching Redox Chemistry
Yue-Ling
Wong, Wake Forest University
Angela G. King, Wake Forest University
Abstract
Application
and development of interactive tutorials and exercises for redox (reduction-oxidation)
chemistry are discussed in this article. These tutorials and exercises
are delivered as Macromedia Shockwave files on the Web. The tutorials
and the practice problems were developed and used by the instructor as
a classroom presentation and by students as outside class review.
1. Introduction
Redox (reduction-oxidation)
chemistry is an important topic of general chemistry. The main aspects
of redox chemistry are assigning oxidation numbers, identifying oxidizing
and reducing agents and redox reactions, and balancing redox equations.
There are well-established sets of rules for assigning oxidation numbers
and identifying redox agents and reactions. As for balancing a redox equation,
specific stepwise procedures are listed in every general chemistry textbook.
Redox chemistry is, however, generally one of the most difficult subjects for students, who often have trouble applying the given rules to specific problems. To master the subject, students need to work through a number of practice problems themselves. The more they practice, the better they understand what they are doing. But a key to their success is that they be given consistent feedback on their work. Our experience has shown that immediate and well-thought out feedback can be of significant benefit in helping students master redox chemistry.
2. Goals
Our discussions
concerning students' difficulty in learning redox chemistry had led us
to conclude that we needed to give them more practice problems with instant
and well-directed feedback. We had looked at most, if not all, of the
chemistry Internet resources, commercial software packages and learning
aids available. None of the Web resources or the software packages reviewed
appeared to satisfy our needs or match what we had in mind. Most learning
aids only grade the final equations, without giving enough feedback to
the students for incorrect answers. However, we had found that when the
students do not get the equation balanced, there are many possible ways
that they could have arrived at the wrong answer. We wanted to be able
to let the students track the sources of their mistakes.
We decided to develop an interactive online tutorial as a learning aid for the students. The goals for the tutorial were:
- to present background material and rules in a clear concise manner
- to provide worked stepwise examples of each type of redox problem, requiring student input
- to present the material in an engaging manner so students will be willing to work problems
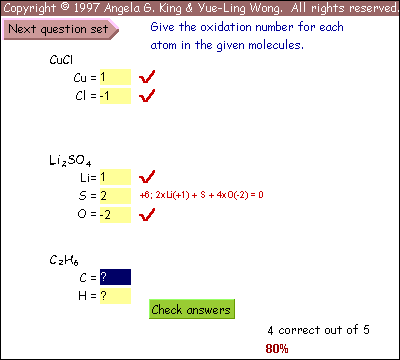
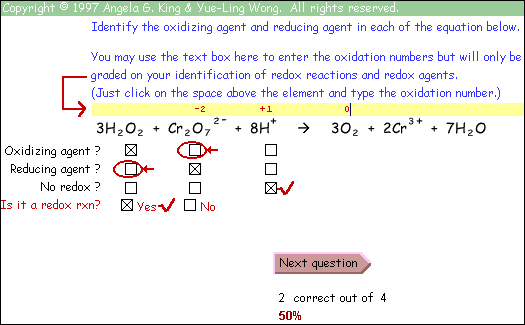
- to provide practice problems with instantaneous feedback on performance
- to mimic grading on exams so students may find their errors
Every sub-topic includes both a tutorial and a practice problem set. The tutorial uses examples to demonstrate the application of the rules. The problems give the students plenty of practice with instantaneous feedback on their answers, so the students know whether their approach to the problems is correct.
3. Program Design
and Usage
3.1
Tutorials
Most of
the online tutorials and existing commercial software were designed for
the students' after-class review. However, when we started creating the
interactive tutorials, our ideas were to make the interactive tutorials
useful as both classroom presentation for the instructor and after-class
review for the students. The goal of making the tutorial for classroom
presentation was not simply to create fancy multimedia presentations or,
like most PowerPoint slides, to save the instructor the time of writing
lecture notes on the chalkboard. Most instructors recognize the problem
of students frantically copying notes from the chalkboard and thus not
listening to the instructor in class. The main idea of creating such interactive
tutorials is to free the students from mindless notetaking and let them
follow closely and listen to the instructor in the classroom.
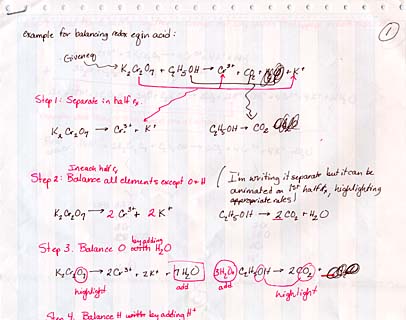
The tutorials were developed based on the lecture notes written by Dr. Angela King. Notes were modified to show how material would be presented in class. These stepwise notes, coupled with comments on design, were used as the basis for the tutorials.
![]()